Abstract
SKY92 combined with ISS identifies high and low risk multiple myeloma in GIMEMA-MMY-3006 VTD arm
Erik H. Van Beers*, Carolina Terragna*, Rowan Kuiper*, Marina Martello*, Elena Zamagni*, Giovanni Martinelli, Martin H. van Vliet*, Michele Cavo*.
Introduction: Prior to this cohort, SKY92 demonstrated significance in several bortezomib-containing treatments (HOVON-65/GMMG-HD4, APEX, TT3, TT6) and thalidomide-containing treatments (HOVON-65/GMMG-HD4, HOVON-87/NMSG-18, TT2, TT3, TT6, MRC-IX). It remains unknown how this GEP-signature predicts outcome in IMiD plus proteasome inhibitor (PI) treatment. GIMEMA-MMY-3006 is the first dataset in which the front-line VTD in NDMM patients has been evaluated by SKY92 and other prognostic markers.
Methods: The GIMEMA-MMY-3006 trial (NCT01134484) compared bortezomib-thalidomide-dexamethasone (VTD) versus thalidomide-dexamethasone (TD) as induction therapy prior to the autologous stem cell transplantation (ASCT) in transplant eligible NDMM patients [1]. The current analysis compares prognostic value of t(4;14), del(13), del(17), GEP and clinical characteristics in the VTD arm (GSE58133; n=114). PFS and OS were evaluated at median follow up of 3.3yrs. GEP data (U133Plus2.0 CEL files) were batch processed and GEP classifiers were calculated as reported [2]. R-ISS was constructed as described in Palumbo et al. [3], except for the missing marker t(14;16) while SKY92-ISS was constructed as described in Kuiper et al. [4] except that four classes were reduced to three (high, low, and the rest).
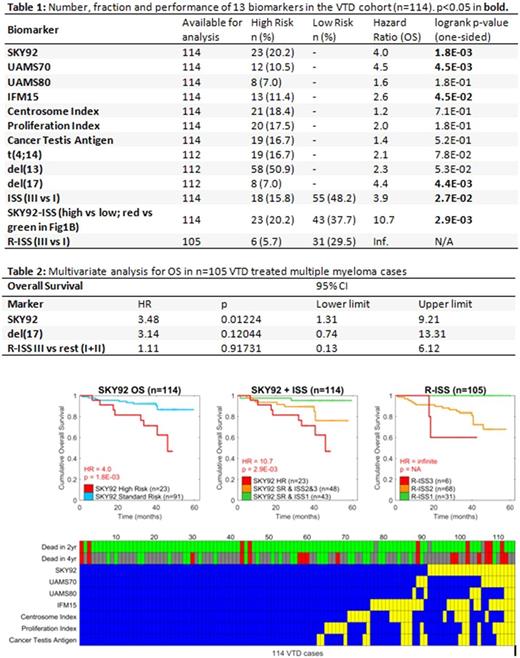
Results: This is the first report of SKY92 evaluated in a VTD setting. The prognostic values of three risk models (ISS, SKY92-ISS and R-ISS) were compared (Table 1). A total of 36 progressions and 18 deaths occurred in 114 cases. Univariate PFS (results will be presented at the meeting). For OS, univariate analysis showed SKY92, UAMS70, IFM15, del(17), ISS (IIIvsI) and SKY92-ISS were significant. Figure 1 shows Kaplan Meier analyses for SKY92, SKY92-ISS, and R-ISS. Multivariate analysis for OS - with SKY92, del(17) and R-ISS (high risk vs others) - significance was observerd for SKY92 (HR 3.48) but not for del(17) or R-ISS (Table 2). Among the 31 R-ISS-I there were no deaths and therefore no HR (between I and III) was calculated (Figure 1C). A comparison of Figure 1B and C shows that SKY92-ISS captured more high-risk patients than R-ISS (23 vs.6). Of the 19 t(4;14) cases, eleven were also SKY92 high-risk. Of the 8 del(17) cases, 5 were also SKY92 high-risk.
Both SKY92-ISS and R-ISS identified low risk patients. SKY92-ISS identified 12 more low risk cases than R-ISS (43/114, 38%, 31/105, 30%, respectively). Figure 1D shows concordance between the risk scores and association with OS (top rows) at 2 and 4y after diagnosis. None of the signatures predicted all deaths at 2 or 4y. SKY92 predicted more deaths than other markers (Figure 1D). Similar to a previous study [1] the risk models were concordant for 2 high risk and 66 standard risk cases, while discordant for 46 remaining cases. SKY92 best identified hazard for a large proportion of cases (Table 1).
Conclusions: In this upfront VTD setting we observe proportions of SKY92 high risk and low risk myeloma that are comparable with previous studies with other treatments containing either bortezomib or thalidomide. SKY92-ISS identifies high risk in 20% and low risk in 38% MM patients with HR = 4.0 between high and standard risk (Fig 1A) and HR = 10.7 between high and low risk (Fig 1B) compared with R-ISS the obtained HR = infinite as there were no deaths in low risk (Fig 1C). SKY92-ISS identified a larger faction of low risk cases (38%) compared with R-ISS (30%) yet retains a very large and significant hazard ratio of 10.7 reproducing earlier validation data [1].
Figure 1. Prognostic marker performance in n=114 VTD treated multiple myeloma patients. Kaplan Meier analyses for OS were stratified by A) SKY92, B) SKY92-ISS or C) R-ISS. D) shows GEP signature predictions (rows) and their overlap across all 114 cases (columns). Standard Risk (blue), High Risk (yellow), Alive at follow up (Green), dead at follow up (Red), lost to follow up (Grey).
References
1. Cavo M et al. Lancet. 2010 Dec 18;376(9758):2075-85.
2. van Beers EH, et al. Clin Lymphoma Myeloma Leuk. 2017 Jul 4. pii: S2152-2650(17)30278-1
3. Palumbo A. et al. J Clin Oncol. 2015 Sep 10;33(26):2863-9
4. Kuiper R. et al. Blood. 2015 Oct 22;126(17):1996-2004
Van Beers: SkylineDx: Employment. van Vliet: SkylineDx: Employment. Martinelli: Amgen: Consultancy; Johnson&Johnson: Consultancy; Pfizer: Consultancy; Celgene: Consultancy; Ariad/Incyte: Consultancy; Roche: Consultancy. Kuiper: SkylineDx: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal